From wound care to neurosurgery – collagen in medicine
Medical products based on collagen are innovative solutions in regenerative medicine. They use the natural protein collagen, which is an essential component of skin, bone and tissues. These products are used to promote the healing of wounds, regenerate damaged tissue and provide structural support. Collagen-based medical devices are well tolerated by the body and provide a biocompatible platform for tissue regeneration. They play an important role in various medical applications, from wound care to neurosurgery.
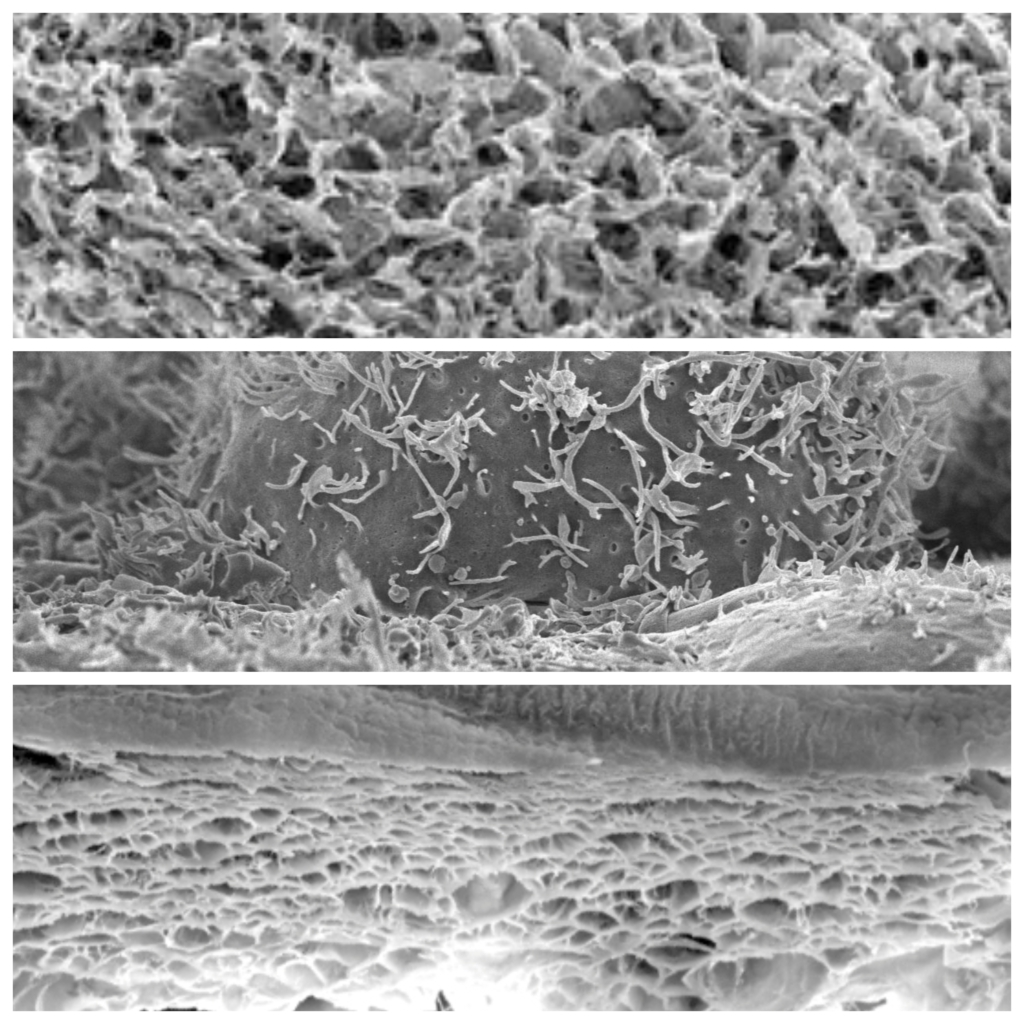
Wound healing with exogenous collagen is a process in which processed collagen material from a natural, porcine source is applied to a wound to promote healing. This is done by providing a structurally stable scaffold that supports cells and tissues as they regenerate.
Collagen as structural scaffold
Exogenous collagen serves as an artificial scaffold surrounding the wound. It provides a stable matrix that offers the cells a firm substrate to attach and grow.
Promotion of cell migration
The exogenous collagen facilitates the migration of various cell types, including fibroblasts, endothelial cells and epithelial cells, into the wound. These cells are crucial for the formation of new tissue.
Supports the formation of blood vessels
Collagen promotes angiogenesis, the formation of new blood vessels. This is crucial to improve oxygen supply to the wound area and transport nutrients necessary for healing.
Promotion of collagen synthesis
Exogenous collagen can stimulate the production of endogenous collagen in the wound. This is important to strengthen the newly formed tissue and restore structural integrity.
Minimization of scar formation
By providing a structurally stable scaffold and promoting targeted cell migration, the application of exogenous collagen can help minimize excess scar tissue.
Modulation of the inflammatory process
Exogenous collagen can influence the inflammatory process in the wound by promoting the release of anti-inflammatory factors.
Moisture control
Collagen wound dressings can help maintain a moist wound environment, which is critical for effective wound healing.
Stabilization of the wound bed
Exogenously added collagen can stabilize the wound bed and promote healing, especially in chronic wounds that often have an inadequate extracellular matrix.
- Demidova-Rice, T. N., Hamblin, M. R., & Herman, I. M. (2012). Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 1: Normal and chronic wounds: Biology, causes, and approaches to care. Advances in Skin & Wound Care, 25(7), 304-314.
- Diegelmann, R. F., & Evans, M. C. (2004). Wound healing: An overview of acute, fibrotic and delayed healing. Frontiers in Bioscience, 9, 283-289.
- Kim, M. S., & Kim, Y. K. (2015). Therapeutic effect of type II collagen on osteoarthritis. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 103(2), 319-326.
- McGuire, J., Li, Q. (2010). Collagen-based wound dressings for the treatment of diabetes-related foot ulcers: A systematic review. Diabetes/Metabolism Research and Reviews, 26(6), 418-431.
- Schultz, G. S., Davidson, J. M., Kirsner, R. S., Bornstein, P., & Herman, I. M. (2011). Dynamic reciprocity in the wound microenvironment. Wound Repair and Regeneration, 19(2), 134-148.
- Singer, A. J., & Clark, R. A. (1999). Cutaneous wound healing. New England Journal of Medicine, 341(10), 738-746.
Hämostyptika aus reinem Kollagen sind spezielle Produkte, die verwendet werden, um Blutungen zu stoppen. Sie basieren auf der blutstillenden Eigenschaft von Kollagen, das eine entscheidende Rolle bei der Hämostase spielt.
Collagen as an adhesion surface
Collagen hemostyptics contain collagen in a form that can be easily applied to the wound. The collagen forms an adhesion surface to which platelets can attach.
Blood platelet adhesion
Once the collagen is applied to the wound, the platelets attach to the collagen. This step is called adhesion.
Activation of blood platelets
Adhesion to collagen causes platelet activation. Activated platelets change their shape and release chemical signals.
Formation of the thrombocyte plug
The activated platelets now adhere firmly to each other and form a platelet plug. This clot temporarily closes the wound and stops the blood flow.
Support of the coagulation system
The collagen in hemostyptics can also activate the coagulation system. This contributes to the formation of fibrin and stabilization of the platelet plug.
Stabilization of the blood clot
The resulting stable blood clot permanently seals the wound and forms a solid barrier that prevents blood loss.
Collagen hemostyptics, then, use the ability of collagen to attract and activate platelets to promote the formation of a platelet plug. This plug, along with support of the clotting system, helps stop bleeding and promote wound healing.
- Brass, L. F. (2003). Thrombin and platelet activation. Chest, 124(3), 18S-25S.
- Davie, E. W., & Ratnoff, O. D. (1964). Waterfall sequence for intrinsic blood clotting. Science, 145(3638), 1310-1312.
- Frimberger, E., Frank, E., Painsipp, E., & Saria, A. (2003). Blood platelets contain and secrete the α1 chain of type I collagen. The Biochemical journal, 376(Pt 1), 65–70.
- Jackson, S. P., & Schoenwaelder, S. M. (2007). Progtaglandins, platelets and thrombosis. Thrombosis and Haemostasis, 97(02), 187-190.
- Monroe, D. M., & Hoffman, M. (2006). The clotting system – A major player in wound healing. Haemophilia, 12(Suppl 3), 11-16.
- Weisel, J. W., & Litvinov, R. I. (2017). Fibrin Formation, Structure and Properties. Subcellular Biochemistry, 82, 405-456.
Ein temporärer Hautersatz aus gefriergetrockneter Schweinehaut wird in der Medizin als vorübergehender Ersatz für verletzte oder verbrannte Haut verwendet.
Biological similarity to human skin
Freeze-dried pig skin has a similar structure and composition to human skin, making it a suitable temporary substitute.
Biocompatibility
Collagen derived from pig skin is biocompatible, which means that it is well tolerated by the human body without triggering a strong immune reaction.
Protection of the wound
The temporary skin substitute protects the injured or burned skin from external influences such as infection and fluid loss and allows undisturbed healing.
Promotion of wound healing
The temporary skin replacement derived from porcine skin promotes wound healing by creating an optimal environment for skin cell migration and proliferation.
Reduction of pain and inflammation
The temporary skin replacement can reduce pain and decrease the inflammatory response in the wound.
- Bates-Jensen, B. M. (1996). Wound measurement. Journal of Wound, Ostomy, and Continence Nursing, 23(6), 282-288.
- Eriksson, E., & Aronsen, K. F. (1981). Selection of a skin bank allograft for burn wound coverage. Plastic and Reconstructive Surgery, 68(3), 422-427.
- Hicok, K. C., Thomas, T., Gruessner, R., & Korbutt, G. S. (2010). Effect of Cryopreservation on human adipose tissue and isolated stromal vascular fraction cells: in vitro and in vivo analyses. Plastic and Reconstructive Surgery, 126(3), 949-958.
- Robson, M. C., Krizek, T. J., & Heggers, J. P. (1973). Biology of Surgical Infections. W.B. Saunders
- Woodroof, E. A., & Clarkson, J. R. (1962). The Use of Homografts of Freeze-Dried Skin in the Treatment of Burns. Annals of Surgery, 155(2), 241–245.
ADM stands for “Acellular Dermal Matrix”. Porcine ADMs (porcine acellular dermal matrices) are biological implants derived from porcine skin and are used in reconstructive and regenerative medicine to promote tissue healing and regeneration. ADMs are a significant tool in reconstructive and regenerative medicine.
Manufacturing
ADMs are produced by removing the cellular components, leaving only the extracellular matrix (ECM). This acellular matrix provides a structural basis for the recipient’s cells.
Promotion of cell adhesion and proliferation
The extracellular matrix in porcine ADMs promotes adhesion of endogenous cells, especially fibroblasts. This supports the formation of new tissue.
Stimulation of tissue regeneration
Porcine ADMs promote tissue regeneration, especially in areas where natural regenerative capacity is limited. They provide a structurally stable foundation for the recipient’s cells.
Promotion of vascularization
The extracellular matrix of porcine ADMs contains signaling molecules that can stimulate the formation of new blood vessels (angiogenesis), which is crucial for supplying nutrients to regenerating tissues.
Minimization of scar formation
By providing a structurally stable foundation, the use of ADMs can help minimize excess scar tissue.
- Badylak, S. F., & Gilbert, T. W. (2008). Immune response to biologic scaffold materials. Seminars in Immunology, 20(2), 109-116.
- Kim, D. E., & Kim, H. S. (2016). Decellularized extracellular matrix-based biomaterials for angiogenesis. Journal of Tissue Engineering and Regenerative Medicine, 10(11), 925-938.
- Nandini, T. (2019). Decellularized dermal matrices in tissue engineering: A versatile platform with high tunability to suit tissue-specific requirements. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 107(7), 2538-2560.
- Turner, A. E., Yu, C., Bianco, J., Watkins, J. F., Flynn, L. E. (2015). The performance of decellularized adipose tissue microcarriers as an inductive substrate for human adipose-derived stem cells. Biomaterials, 73, 31-42.
In der Orthopädie werden Kollagenmembranen als Barrieremembranen eingesetzt, um Gewebe zu trennen und zu schützen, während die Regeneration von Knochen und Weichgewebe stattfindet.
Biocompatibility and resorption
Collagen membranes are made of biocompatible collagen material and are well tolerated by the body. They are absorbable, which means that they resorbe naturally over time.
Barrier function
Collagen membranes serve as a mechanical barrier between different tissues, for example between bone and soft tissue. This barrier prevents the penetration of soft tissue into the wound and thus enables undisturbed regeneration of the hard tissue.
Promotion of cell adhesion and proliferation
Collagen membranes promote the adhesion of cells, such as osteoblasts (bone cells), and support their proliferation. This is crucial for the formation of new bone and soft tissue..
Controlled soft tissue growth
Collagen membranes prevent uncontrolled growth of soft tissue into the regenerating zone, which favors the development of hard tissue.
- Giannoudis, P. V., Faour, O., Goff, T., & Kanakaris, N. (2007). Determinants of the optimal time for the administration of autologous bone marrow cells in the management of fracture non-unions. Injury, 38(Suppl 2), S100-S107.
- He, C., & Jin, Y. (2010). Novel method for the purification of collagen using organic electrolytes and low speed centrifugation. Bioresource Technology, 101(24), 9557-9562.
- Salgado, A. J., Coutinho, O. P., & Reis, R. L. (2004). Bone tissue engineering: State of the art and future trends. Macromolecular bioscience, 4(8), 743-765.
- Suh, D. Y., Singh, J., Abatelyan, A., & Dendorfer, A. (2008). Inflammatory response and biodegradation of dermal sheep collagen cross-linked using a combination of chemical and gamma irradiation methods. Journal of Biomedical Materials Research Part A, 84(3), 646-656.
Die Resorption von implantiertem Kollagen ist ein wichtiger Prozess, der bestimmt, wie lange das Kollagenmaterial im Körper verbleibt, bevor es abgebaut und durch körpereigene Substanzen ersetzt wird
Enzymatic degradation
Collagen is degraded by specialized enzymes, especially collagenases. These enzymes split the collagen molecules into smaller fragments.
Phagocytosis by macrophages
The degraded collagen fragments are phagocytosed by macrophages, i.e. taken up and degraded by these cells.
Tissue remodeling
The resorption of collagen enables the remodeling of the surrounding tissue. This is particularly important in wound healing and in the formation of new tissue.
Metabolite excretion
The degraded collagen fragments are excreted in the form of metabolites that are easily metabolized and eliminated by the body.
Es ist wichtig zu beachten, dass die Geschwindigkeit der Kollagenresorption von verschiedenen Faktoren beeinflusst wird, einschließlich der Art des implantierten Kollagenmaterials, des Implantationsortes und der individuellen physiologischen Bedingungen des Patienten. Die Resorption von implantiertem Kollagen ist ein natürlicher Teil des Heilungsprozesses und ermöglicht die Integration des körperfremden Materials in das umgebende Gewebe.
- Eming, S. A., Martin, P., & Tomic-Canic, M. (2014). Wound repair and regeneration: mechanisms, signaling, and translation. Science Translational Medicine, 6(265), 265sr6.
- Nagase, H., Visse, R., & Murphy, G. (2006). Structure and function of matrix metalloproteinases and TIMPs. Cardiovascular Research, 69(3), 562-573.
- Ricard-Blum, S. (2011). The collagen family. Cold Spring Harbor Perspectives in Biology, 3(1), a004978.
- Wynn, T. A., & Vannella, K. M. (2016). Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity, 44(3), 450-462.
